Metal Foams & how to make them
 This is a quick guide to making metal foams - mostly aluminium foams. It's drawn from one of the introduction chapters of my 2004 PhD thesis (the full version, with sources and experimental processes, is free to download here). There are lots of different ways of making foams, with different levels of complexity. Aluminium is the most commonly made foam, because it's light and easy to work with - though some of these processes can work with other metals.
This is a quick guide to making metal foams - mostly aluminium foams. It's drawn from one of the introduction chapters of my 2004 PhD thesis (the full version, with sources and experimental processes, is free to download here). There are lots of different ways of making foams, with different levels of complexity. Aluminium is the most commonly made foam, because it's light and easy to work with - though some of these processes can work with other metals. - The level of porosity of the foam (described here by the ratio of the density of the foam * to that of the solid metal s, where */s numbers towards zero are very light foams, and numbers towards 1 are more or less solid metal with the odd bubble in it)
- The sort of process used (how fast it works, whether it can make occasional tiny samples slowly or churn out loads of foam on a continuous basis)
- The sort of foam that each process makes (are the cells open - more of a framework - or closed; how small are the cells, and how regular is the general structure)
- The regularity of the end product (is the foam reliable and uniform, or an unpredictable material where every sample is different?). If the production process is sensitive to slight variations in processing parameters it is likely to produce less uniform foams, susceptible to the presence of flaws and defects.
- The microstructure of the parent metal is also important, with some processes imposing restrictions on the metals or alloys which can be used, or requiring the addition of other materials which can have a deleterious effect on mechanical properties.
Making closed-cell metal foams by directly adding gas to metals
The simplest method of foaming a liquid melt involves direct injection of bubbles of gas. The key requirements are for a method of producing and efficiently dispersing fine bubbles, and of keeping them in place long enough for solidification to occur before they escape. The foaming gas is dispersed with a rotating impeller. Bubbles rise to the melt surface, and are drawn out on a conveyor belt, gently rolled while still partly molten to produce a smooth surface, and cooled. Continuous sheet of solid foam is produced, as shown below. This type of process has been commercialised by Norsk Hydro™, Alcan™ and Cymat™ Corporation, and allows for the continuous production of foam sheets -
The foams have a fairly irregular structure, since the volume fraction of refractory particles that can be added is limited to levels that do not completely eliminate melt drainage and cell coalescence. The process relies on a relatively low viscosity for efficient dispersion of the gas bubbles from the impeller, limiting the extent to which the viscosity can be raised. Stirring of the melt to aid gas dispersal is possible, but can destroy the foam structure by destroying the molten walls between adjacent bubbles.
Making closed-cell metal foams by adding a foaming agent to a thickened melt
Rather than bubbling gas directly to molten metal, a powdered chemical foaming agent, which is stable at room temperature but releases a gas at the melt temperature, can be stirred into the melt. Titanium hydride (TiH2), the only foaming agent used commercially, decomposes to titanium and hydrogen gas at temperatures above ~ 400ºC by the reaction:
TiH2(s) → Ti(s) + H2(g) The foaming agent can be added directly to molten metal, as illustrated below. Provided the powder can be dispersed evenly before the onset of decomposition, the hydrogen released forms pores throughout the metal. As with the direct addition of gas, it is necessary to stabilise the molten foam against collapse, which is usually achieved by adding a reactive alloying element such as calcium or magnesium to the melt, and stirring it vigorously while air is bubbled through it. This raises the viscosity by generating a dispersion of fine solid oxide particles in situ. |
This process is used commercially to produce Alporas™ aluminium foams. Air is stirred into a melt containing 1.5 wt.% Ca for 6 minutes at 680‑720ºC, forming fine solid particles of CaO and CaAlO4. 1.6 wt.% of TiH2 is then added to the melt, which is held at 680ºC for 4‑15 minutes. The initial gas produced is mostly burnt off, but the foam then expands. A ductile and relatively uniform cell structure is obtained in batches of up to 160 kg, with */s of 0.05‑0.3 and cell size of 2-10 mm.
One drawback is the high cost of the foaming agent and the calcium. Although effective at producing large blocks of foam in regular shapes, the requirement for stirring of the full volume to disperse the foaming agent throughout the volume also means the process cannot produce complex net-shape components. Attempts have been made to develop injection techniques using metallic foams, whereby a melt is injected into a die as it foams, but with only limited success: heating and cooling rates are difficult to control across the sample, and the injection pressure is necessarily low to avoid crushing the molten foam, favouring irregular cell structures with density markedly decreasing with distance from the injection point.
This is an optical cross section of an Alporas™ Al‑1.5wt.% Ca foam -
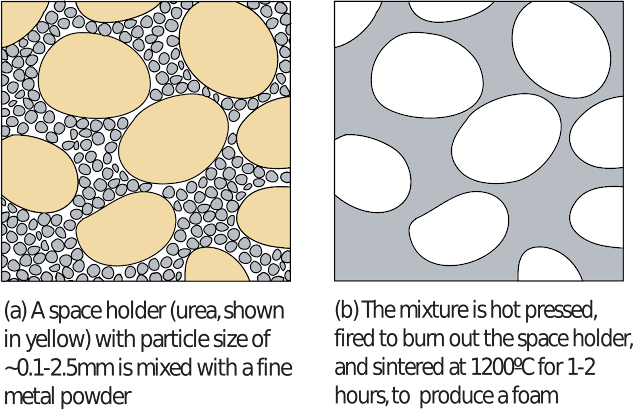
Making closed-cell metal foams with a 'foamable' precursor made from metal powder
The use of chemical foaming agents provides more control over the final cell structure than the direct injection of a gas. However, it remains relatively difficult to control the foaming process, given the requirement for full powder dispersion. Several processes have therefore been developed which separate the dispersion of the foaming agent within the melt from the decomposition of the agent to form a foam. Typically, powdered metals are mixed with a foaming agent at room temperature, and compacted to form a 'foamable precursor' material, which is subsequently 'baked' by heating it inside a shaped mould above the metal melting temperature to form a foam.
This provides advantages over a 'single step' process. Since foaming is not taking place during mixing, the powdered foaming agent can be thoroughly mixed in the metal, meaning that finer powders can be used, and the powder can be more uniformly dispersed. A foamable precursor is produced which can be stored indefinitely, and cut sections of it can be foamed in moulds with complicated shapes, without the need for access by a stirrer, to produce net-shape components with a solid outer skin. Whereas in the direct addition of a foaming agent to a melt, the temperature at which foaming takes place is restricted by the need to accommodate the stirring of the powder into the melt prior to full thermal decomposition, in a two stage process the foaming temperature and time, and hence the porosity and cell structure, can be precisely tailored to requirements.
The foamable precursor must be impermeable to gases, or any gas produced can simply escape, meaning some form of consolidation is required to fuse the mixed powders. Various routes to consolidation are illustrated below. Extrusion, at 440ºC and ~ 80 MPa, generates sufficient localised friction between the particles to break up their surface oxide film and bond them together. Preliminary compaction by cold isostatic pressing can facilitate extrusion. As an alternative to extrusion, the powder mixtures can be consolidated by hot isostatic pressing at 400-450ºC, sometimes with preliminary cold pressing to facilitate handling. The powder particles are joined together primarily by diffusion. This process apparently allows foaming agents which decompose at temperatures under 400ºC to be used, as the high pressure prevents premature thermal decomposition.
Although surfactant or viscosity enhancing agents can easily be added to the initial powder mixture, they are not normally required, since the large surface area of the gas-atomised metal powders used is covered with a sufficient quantity of native oxide to stabilise the liquid foams against collapse.
Such processes have been used to foam aluminium, bronze, copper, steel, lead and magnesium, and are used commercially to produce Foaminal™, Alulight™ and Fraunhofer™ foams of various Al-Mg-Si alloys with */s of 0.11‑0.37 and cell sizes of the order of millimetres. The cell structure of the foams can be carefully controlled by adjusting the time and temperature at which the foamable precursor is foamed, in contrast to processes where TiH2 is added directly to melts.
This diagram illustrates processing routes used in the production of foams based on powder metallurgy, an aluminium foam produced using a powder precursor -
The picture below is a a shaped component, with a rather irregular cell structure, produced by baking a foamable precursor in a shaped mould -
Metal foams made using 'foamablr precursors' made from molten metal
In the FORMGRIP process (Foaming of reinforced metal by gas release in precursors), recently developed by Gergely and co-workers, a foamable precursor is produced using molten metal. The foaming agent titanium hydride, TiH2, is subjected to a preliminary thermal heat treatment – typically for 24 hours at 400ºC, followed by one hour at 500ºC – to generate a TiO2 diffusion barrier on the surface. This delays the evolution of hydrogen sufficiently for the TiH2 to be dispersed thoroughly into an aluminium melt, and the melt solidified, before significant foaming takes place. The foaming agent is mixed with powdered aluminium in the weight ratio 1:4 prior to addition, to facilitate the mixing process. In order to stabilise the molten foam, ~ 10 vol.% of particulate SiC is added to the melt in a preliminary step. The resulting foamable precursor material has */s of ~ 0.75, due to gas incorporated during the mixing stage and limited decomposition of the hydride before the metal was solidified.
The precursor is then baked to form foams with */s of 0.1‑0.3 and cell size of 2‑8 mm. A key advantage of the process is that - since the mixing and foaming stages are independent - the porosity and cell size can be closely controlled by choosing appropriate time and temperature of foaming.
This is a schematic illustration of the FORMGRIP foaming process -
This process combines the low cost of melt-based processing with the enhanced control over the foaming process associated with foamable precursors. Complex moulds can be filled with foam, and ceramic components have been incorporated into foam castings. Drawbacks with the process are the requirement for a lengthy heat treatment, and the relatively high cost and hazardous nature of titanium hydride (which is pyrophoric when in fine powdered form).
Making open-cell foams by replicating a polymeric foam structure
The relative ease of producing high-porosity open-celled polymeric foams with uniform cell structures has prompted their use as templates in the production of metallic foams by two-stage investment casting processes. As shown in the diagram below, the polymer – generally polyurethane – is infiltrated with plaster and fired to burn out the polymer. The mould is then filled with molten metal, often aided by combinations of vacuum and external pressure, and the plaster is dissolved.
This is a typical ™ foam produced by ERG Inc -
Foams can be produced with a wide range of metals and alloys, and these foams can be very light with */s as low as 0.03. This process shares many of the advantages and disadvantages of processes employing salt templates. The lower limit of cell size, and the upper limit of batch size, are similarly constrained by the need for full infiltration of a tortuous mould, and for full dissolution of the plaster. Areas of varying porosity, or even solid metal, can be incorporated as the polymer mould is assembled. Duocel™ foams produced by this method are used as heat exchangers, liquid and gas baffles, high-purity porous electrodes, and chemical filters.
Making open-cell foams by investment casting, with disposable templates of the cell structure
A variety of casting processes, mainly used in the production of open-cell foams, are based on the use of disposable templates of the cell structure. In the earliest such process, coarse rock salt (NaCl) was sintered in air for a few hours to fuse the grains together, effectively forming a mould of an open cell foam. Molten metal was then poured in to infiltrate the pores, and the salt dissolved to produce an open-cell foam with cell size of ~ 3‑4 mm.
The use of hot-wall infiltration, which reduces the tendency of the metal to solidify before full infiltration is achieved, and of combinations of partial vacuum and high pressure applied during the infiltration process, have recently made it possible to produce far finer-scale structures, as shown below. The foams thus produced have cell sizes as small as 400 µm, with */s of 0.2‑0.3.
The photo below is a SEM micrograph of a typical foam produced.
Infiltration processes do not require expensive metallic powders or fibres, and almost any alloy can be used provided it melts at a temperature below that where the template melts. As the elements forming the mould can be carefully controlled, the foams produced are characterised by a high degree of structural uniformity. The process is relatively complex, requiring extended sintering and dissolution. The lower limit of cell size and the upper limit of batch size obtainable are determined by the need for full infiltration of often tortuous templates and dissolution of the salt in a reasonable time.
In a variant of the process, shown in the diagram below, powdered metal has been mixed with powdered salt and compacted to form a dense block. This is then sintered, above or below the melting temperature of the metal (but below that of the salt), followed by dissolution of the salt, producing foams with */s over 0.2. The process is unlikely to find widespread use, as it combines the complexity of the pressure infiltration process with an additional requirement for relatively costly powdered metal. It appears to produce fewer connections between the salt grains, leading to a denser, less porous structure, containing residual NaCl.
This is a SEM micrograph of a foam thus produced -
Making open-cell foams by sintering metal fibres or powders together
Arguably the simplest method of producing an open-celled foam consists of sintering powdered metals, usually produced by gas atomisation, at a temperature close to their melting point to fuse the particles together to produce a material with interconnected porosity. Sintering is often carried out in a vacuum or inert atmosphere to prevent surface oxidation, and typically takes 1‑2 hours. The porosity of the end product, shown in the diagram below, depends on the packing efficiency of the powder. It is usually low, with relative density – defined as the ratio of the density of the foam, *, to that of the metal, s, between 0.5 and 0.8. Lower density can be obtained by mixing particles of a volatile fugitive material with the powder, which evaporates or decomposes at the sintering temperature to leave additional voids in the structure. Chemical foaming agents which release a gas at high temperatures can also raise the porosity, if sintering temperatures are sufficiently high to allow limited plastic flow.
These images show porous titanium made by sintering of powders in a reducing atmosphere, and porous stainless steel produced by cold isostatic compression followed by liquid-phase sintering -
Metal fibres pack to give significantly higher porosity than powders (with */s ~ 0.05). This next picture is a SEM micrograph of a porous material produced by sintering extruded stainless steel fibres -
And these are a SEM micrograph of a porous material produced by sintering drawn stainless steel fibres, and an enlarged view of a sintered joint between two fibres.
This type of process is rapid and energy efficient, with */s ~ 0.45 and cell sizes of under 1 mm obtained. The range of alloys that will react is, however, limited by the need for an appropriate reaction, and the intermetallic compounds can be brittle.
Foams made by sintering hollow spheres
Lower densities can be obtained if hollow powders are used. Gas atomisation of metals inevitably produces a proportion of hollow particles, with diameter under 1 mm, that can be separated from dense powders by a sedimentation process. Larger hollow spheres can be produced by a process illustrated below (production of hollow aluminium spheres using slurry ejection. The bubbles are subsequently sintered to remove the volatile phases).
Hollow 'green' spheres of diameter ~ 4 mm can be made with a slurry containing dispersed metallic particles, a binder, stabilising and dispersing agents, and a continuous volatile phase, and subsequently fired to fuse the metal particles and evaporate the other phases.
They can also be produced directly from molten aluminium, as shown below: Production of hollow aluminium spheres by capillary instability of a gas-cored liquid column. Air and molten metal are ejected concentrically from a die. With careful control of flow parameters separate metal bubbles are produced, which solidify during a long drop in cool air and are collected in a quenching liquid.Hollow sphere foams have recently been produced at lower cost by using spheres of expanded polystyrene as a template for the spheres, as shown below (showing production of a hollow sphere foam using slurry-coated polystyrene spheres as a template.). Sintering the compact burns out the polystyrene, evaporates the volatile component of the slurry and fuses the spheres together. This process offers a slightly lower processing cost, as there is only one heating step, and no requirement for precise flow control. The spheres can be more effectively compacted, improving contact between the spheres.Hollow spheres are bonded together by sintering for up to 24 hours, either in a reducing atmosphere or in air with the spheres coated with a bonding slurry. As the spheres can easily be sorted by size, the foams produced can have highly uniform structures with */s ~ 0.1‑0.2.
Hollow sphere foams have interesting properties, with a controlled mixture of open and closed porosity, and can be produced as a net-shape product with a high degree of uniformity. However the cost and complexity of production make them unlikely to find widespread application.
The next three images are a foam produced by sintering expanded polystyrene spheres coated with a slurry containing stainless steel, a close up of a much finer foam showing necking between stainless steel spheres bonded using a magnetite slurry, and a cross section showing necking between hollow Ni spheres sintered at 1300ºC for 24 hours -
Making closed-cell foams by infiltration of a hollow sphere compact
Hollow spheres can also be used as a template of the cell structure. Ceramic or metallic spheres with diameter as small as ~ 60 µm are loosely packed, sometimes preheated, and infiltrated under relatively low pressure (~ 0.5 bar) with molten metal, a shown in the diagram below. The spheres can be sorted by size and arranged in highly regular structures, or randomly dispersed. The limited packing density which can be achieved with spheres of uniform diameter means */s is usually over 0.4.
Chemical decomposition on a disposable precursor
Open-cell polymer foams can also be used as a deposition template, as shown in the diagram below. A polymeric foam is coated with a substance which absorbs infrared radiation, generally carbon black or appropriate pigments. Radiation locally heats the coated surface, which is exposed to an atmosphere of Ni(CO)4 gas. This decomposes at high temperature to Ni(s) and CO(g) at the hot surface, coating the polymer with nickel. The polymer is subsequently burnt out to produce an open-cell foam with hollow struts.
With no direct restriction on cell size, extremely fine-scale foams can be produced. The range of metals is, however, limited by the need for a suitable deposition reaction. The foams produced, sold under the trade name Incofoam™, are used as filters and porous battery electrodes.
SEM micrographs of an open-cell Incofoam™ nickel foam at two levels of magnification, showing the hollow struts -
Electrodeposition on a disposable precursor
In a similar process, metal is electroplated on to the surface of an open-cell foam. The surface of the polymer is made to conduct, by coating with carbon black or vaporising a thin metal layer on it. It is then electroplated and sintered to remove the polymer. A Celmet™ porous electrode thus produced is shown in the picture below.
Foams made by exploiting chemical characteristics: porous materials by direct deposition
A recent process to extract reactive metals such as Nd, V, Nb and Ti by electro-deoxidation of their oxides in a molten salt solution produces the metals in the form of an extremely fine open-cell foam. The images below show an electrolytic cell for the reduction of pellets of the oxides of reactive metals, and SEM micrograph of an Nb foam produced by electro-deoxidation of Nb2O5.
To produce Ti foams, a voltage of ~ 3 V is applied to the oxide in a bath of molten CaCl2 at 950ºC for 12 hours. Although costly and relatively hazardous, this process can produce foams on an extremely fine scale.
Foams made by directional solidification
The large difference in solubility of hydrogen between liquid and solid metals can be exploited to produce porous metals by the controlled solidification of metal‑gas eutectic systems. In the GASAR process, a high pressure of hydrogen is applied to a molten metal to dissolve gas in the melt. The melt is then transferred to a mould with a cooled base, as shown below, prompting directional eutectic solidification at ~ 5 mm s-1. Gas released at the solidification front nucleates elongated pores, in much the same way as a lamellar structure can be formed in a metal-metal eutectic.
The foams obtained – sometimes called lotus structures – have been produced with Ni, Cu, Al, Fe and Mg, with pore diameter between 10 µm and 5 mm and minimum */s of 0.35. The cell structures are sensitive to fluctuations in pressure and cooling rate. The explosive nature of hydrogen means its use near metal melts is hazardous; however recent results suggest nitrogen is equally suitable for the foaming of iron. The complexity of the production process probably precludes the widespread use of such foams.
Closed-cell Foams made using alloy systems with a volatile phase
In an early process shown in the diagram below, a metal is melted inside a pressure vessel with another metal of significantly lower melting point, and heated to a temperature where the more volatile component will vaporise. The high pressure causes the molten metal to become supersaturated with vapour. The pressure is then suddenly released, prompting the rapid expansion of the volatile phase contained within the molten metal, producing a foam of mostly closed pores with a diameter of 1‑3 mm.
Porosity caused by diffusion
The Kirkendall effect has been used to generate porous materials. Powders of two metals - one of which is chosen to have significantly higher diffusivity than, and solid solubility within, the other - are mixed, compacted, and annealed for 20‑50 hours. The component with the higher diffusivity diffuses preferentially into the other component creating pores. A porous material thus produced is shown in the diagram below. */s of ~ 0.6 has been achieved in Zn-Al and Cu-Ni systems. Severe restrictions on the metals that can be used, and extended processing times, mean that this process has few advantages over powder sintering. This is a porous structure formed by Kirkendall diffusion, containing 73% Zn, 27% Al, formed by annealing an Al‑Zn mixed powder compact for 48 hours -